

I wasn’t at the beginning for the design of the study, but I think that actually if we hadn’t had a crossover with the chemo arm, we may not have recruited many patients at all, and that would have been a real shame. The fact is that everyone wondered why FD allowed it, crossover ethically was it the right thing, it would completely impair overall survival. Okay, so I think we wouldn’t have asked this PFS2 question if there hadn’t been a crossover. So, one question is of course the concept of PFS2 and we’ve used it in other colorectal studies many years ago in the stop-and-go setting, so explain PFS2 to us, what does it mean and what do you want to capture and what really, for the newcomer to this question, why PFS2? Okay, very interesting and very well summarized. The lines do not cross so I do think, for some reason, despite that initial drop in PFS, and we can talk about pseudo progression and things like that, pembro in first line still would remain, from a Phase III randomized trial, the standard of care treatment now for MSI-high advanced bowel cancer. Personally, I think this, for me, means that it’s still a good thing for our patients to have immunotherapy in first line, as long as, there’s all these little bits of, should we sub biomarker patients and clinically biomarker them and stratify them, and that is for discussion, but it looks good. My general take-home message on this is that it’s very promising as an interim analysis because we will get OS survival data in the next, I think, few weeks, or next few months. Now we couldn’t answer all the questions in the chat and I’m sure you’re going to ask me lots of questions.
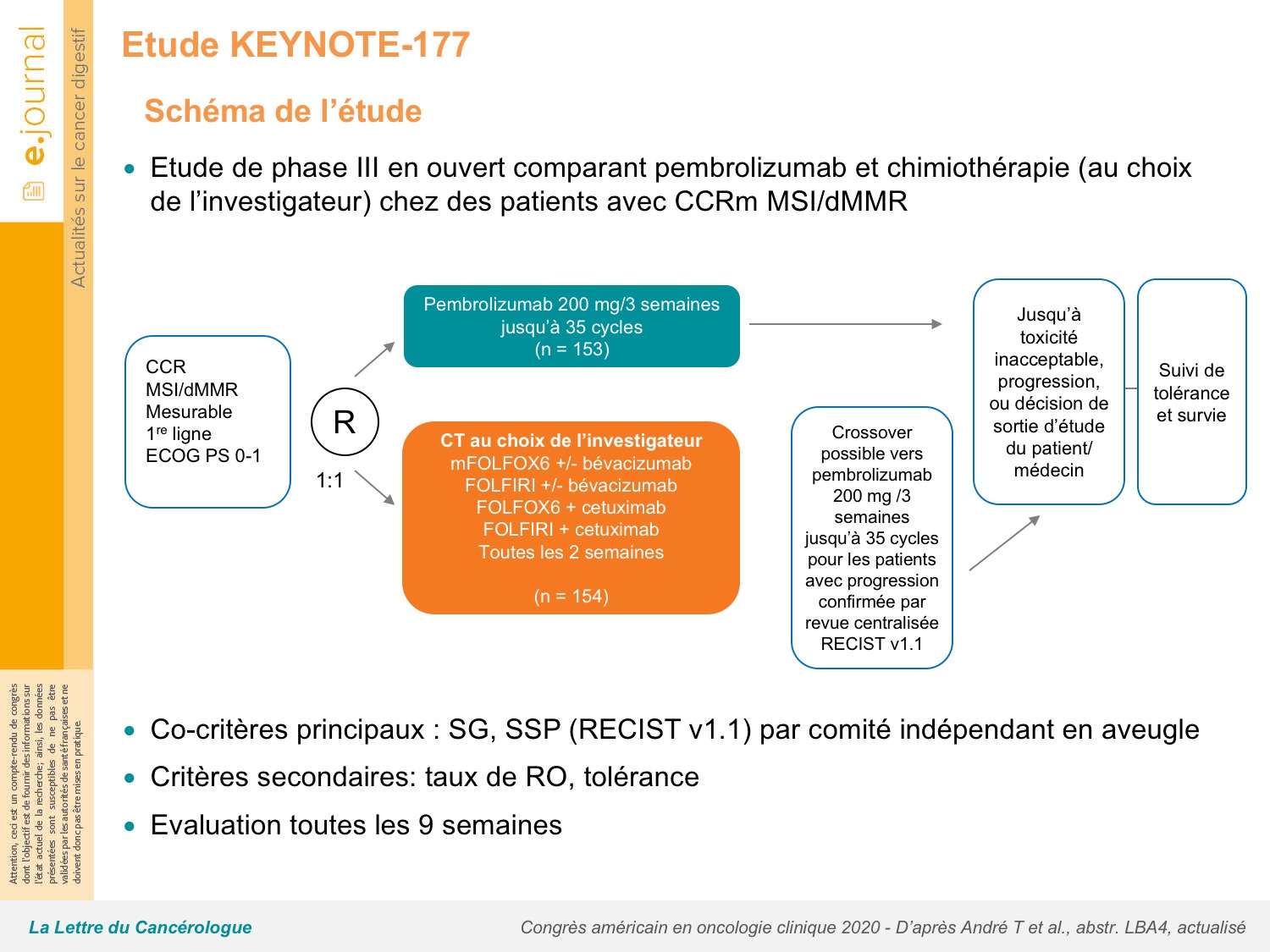
Whereas it was only about 24 months for the chemo first arm. And so actually if you look at those lines, the median PFS2 has not reached is not reached at all, I think it’s 60% of people were non-progressing on their second minor therapy in the pembro first arm. This is very interesting because there was a crossover of around 60% of patients who had some form of immunotherapy either within the study, in the context of the crossover design, or off-study. So, actually, bottom line it showed that the curves continued to separate for PFS2. And that was because of that slightly concerning initial drop in the PFS in the first three to six months. But now, with the crossover, everyone was wondering whether, actually, from a sequencing point of view, is pembro first best, followed by chemo, or chemo first followed by pembro. So this was, we’d already shown that pembrolizumab was better than chemo for PFS1, 16.5 months versus 8.2.
#Keynote 177 trial
And it kind of seemed like a bit under radar because I think no one quite realized when I presented it that we were going to look at this progression-free survival 2 end point, which is important in this trial because there’s a crossover. So, I was honored to present the updated data on this Phase III randomized trial pembrolizumab in first line versus chemotherapy. Thanks, Tobi and thanks to the whole team. Give us a brief flavor, but also what I want to discuss with you are some questions, I will ask you about the outcomes you presented. Kai-Keen, can I start with you? You presented the KEYNOTE-177 data. I’m really excited to discuss with you the latest evidence and, really, also forward-looking evidence and for, hopefully, a successful 2021 in GI cancers. He’s also leading the CAP taskforce here in the U.K. His research interests are GI cancers and he’s also running several trials in GI immunotherapy trials. Hi, Lizzy.Īnd then Kai-Keen Shiu is a colleague, an oncologist at University College, London, with me here in London. Lizzy is SMO GI faculty member and also leads the ERTC GI taskforce. Lizzy is a consultant oncologist, gastrointestinal oncology at Addenbrooke’s Hospital at the University of Cambridge. She is a GI oncologist, particularly interested in upper GI cancers and neuroendocrine cancers, and also member of the NCI GI taskforce.
Let me introduce my esteemed team Nataliya Uboha, who is Assistant Professor at the University of Wisconsin. But before I introduce my colleagues, I want to thank VJOncology to really bring this new format on to discuss take-home messages from GI ASCO. And I’m really excited to, first of all, introduce you today to a team of experts in GI cancers who followed with me, virtually this year, the GI ASCO program. I’m a medical oncologist and interested in GI cancers.

I’m the Executive Medical Director for Sarah Cannon Research Institute.


 0 kommentar(er)
0 kommentar(er)
